As we age, sunlight and environmental stress break down our skin’s underlying support structure. Bone and tissue mass shrink, and youthful facial fat pads thin and migrate downward as gravity takes effect. In addition, the skin makes less collagen (an essential protein for skin support) every year, with a steady 1% decrease annually starting as early as 20.
All of this means that in your 20s, 30s, 40s, 50s, and beyond, age-related volume loss may feel frustrating, causing problems such as:
- Laugh lines (nasolabial folds)
- Cheek thinning and sagging
- Droopy jowls
- Thinning lips and lip lines
- Loss of jawline definition
- Under eye hollows

To help reverse these age-related changes and restore a beautiful, natural appearance, Northshore Dermatology Center conveniently offers the four industry-leading dermal filler options in our offices in Lake Bluff, Libertyville, and Wilmette, IL. Our experienced certified nurse injector, Mellisa Feldman, artistically administers fillers tailored to your unique needs.
Each dermal filler shines uniquely. They can fill fine lines, smooth facial folds, plump volume, gently lift sagging skin, and even provide a natural lit-from-within glow.
Restylane®: Smooth, Hydrate, and Define
Restylane is a hyaluronic acid (HA) filler designed to restore moisture, smooth lines, and add natural-looking volume. HA is a substance your skin naturally produces, but levels decline with age, contributing to wrinkles and sagging. Restylane helps plump lips, soften nasolabial folds, and rejuvenate under-eye hollows. It also enhances skin hydration for a dewy, refreshed look.
With a variety of formulations tailored for different areas, Restylane delivers long-lasting results while keeping your facial expressions natural and dynamic. Restylane formulas usually last 6-18 months based on the formula and injection area.
Juvéderm®: Lift, Contour, and Volumize
Juvéderm, another HA-based filler, is known for its silky-smooth consistency and ability to restore youthful contours. It works by replenishing lost volume, smoothing deep wrinkles, and enhancing facial features. Popular treatment areas include the lips, smile lines, cheeks, and jawline. There's also a specific Juvéderm formula, administered in microdroplets, that provides an instant improvement in skin glow.
Juvéderm’s advanced cross-linked formulas ensure results that can last up to a year or longer, depending on the product used. Based on the treatment area and formula, these fillers typically last 12-24 months.
Radiesse®: Sculpt and Support Your Skin
Radiesse is a calcium hydroxylapatite (CaHA) filler that does more than just add volume—it also stimulates collagen production for firmer, more youthful skin. Ideal for deeper lines and facial contouring, Radiesse is commonly used for lifting cheeks, defining the jawline, and smoothing smile lines.
It’s also a favorite for hand rejuvenation, helping to restore lost fullness and minimize visible veins. Radiesse provides immediate results with long-term benefits, as the CaHA microspheres encourage your skin to produce its own collagen over time. Results last a year or more.
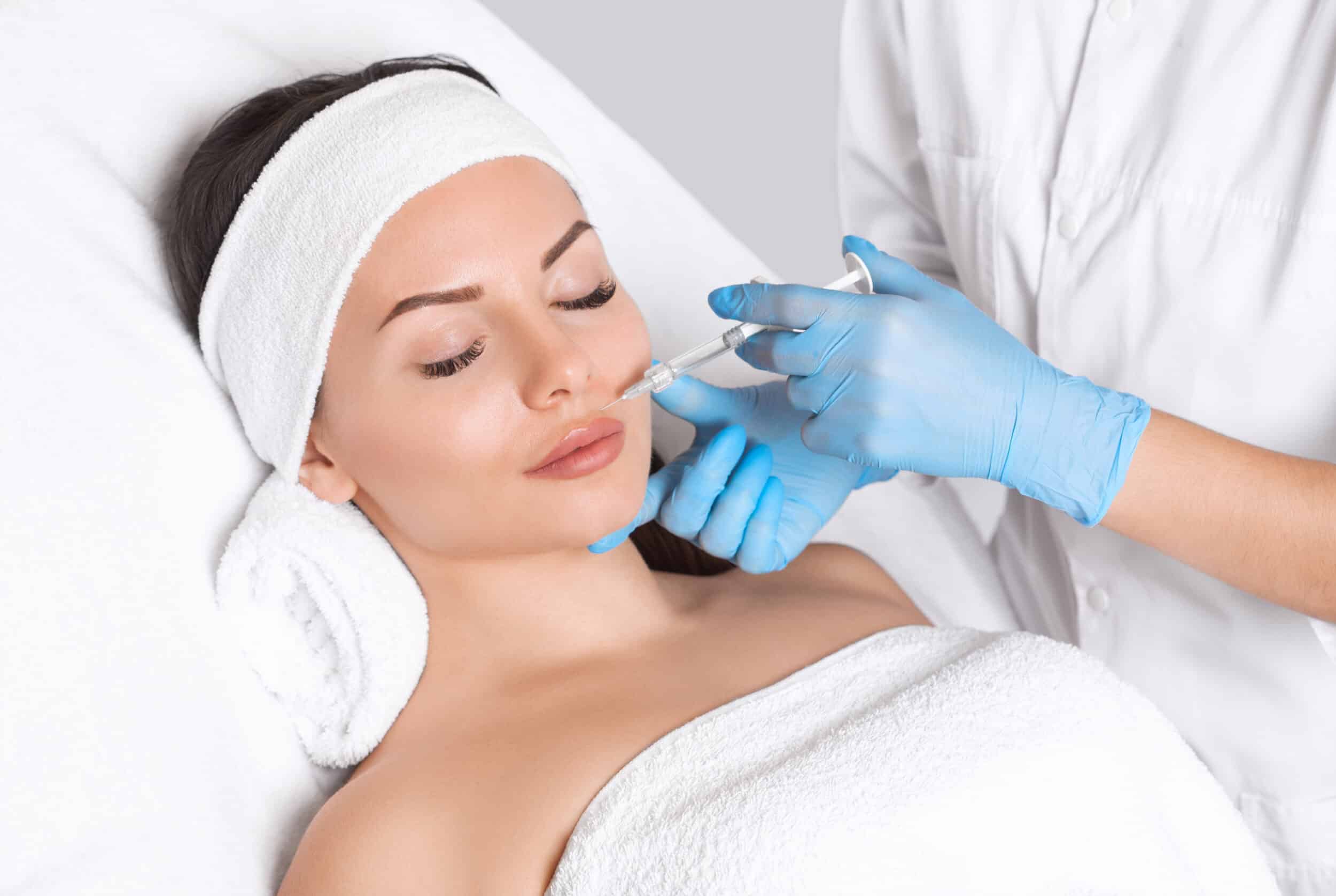
Sculptra®: Gradual Volume, Lasting Glow
Sculptra is an FDA-approved biostimulatory filler made from poly-L-lactic acid, a substance that gradually restores facial volume by stimulating collagen production. Unlike traditional fillers, Sculptra works over time, creating subtle, natural-looking fullness that lasts up to two years. It’s an excellent choice for hollow cheeks, deep wrinkles, and overall skin rejuvenation.
Studies show that Sculptra increases Type 1 collagen by over 65% after three months. Sculptra is also FDA-approved for skin glow. Many patients love the added glow and firmness that come with Sculptra’s collagen-boosting effects, making it perfect for those looking for long-term, youthful skin.
Why Choose Us for Dermal Fillers?
Patients on the North Shore trust us for expert, natural-looking results, thanks to years of specialized filler experience, precision injection techniques, and a warm, patient-first approach. Mellisa Feldman, our certified nurse injector, has been perfecting the art of facial fillers for over seven years, delivering stunning, confidence-boosting outcomes.
Great results start with a great injector-patient relationship. That’s why we take the time to truly understand your aesthetic goals, ensuring your treatment is tailored to your unique features. When you feel heard and supported, your results are not just beautiful — they’re exactly what you envisioned.
Put Your Best Face Forward With Fillers on the North Shore
Whether you’re looking for a quick boost or long-lasting rejuvenation, fillers offer beautiful, natural results. Call Northshore Dermatology Center at 847-234-1177 to book your consultation and let our team help you find the perfect filler for your goals.